
By contrast, in bulk and solution free-radical polymerization, there is a tradeoff between molecular weight and polymerization rate. High molecular weight polymers can be made at fast polymerization rates.Īdvantages of emulsion polymerization include: īatch emulsion polymerization: Emulsion polymerization in which all the ingredients are Note: With the exception of mini-emulsion polymerization, the term “emulsion polymerization”ĭoes not mean that polymerization occurs in the droplets of a monomer emulsion. Resulting in particles of colloidal dimensions containing the formed polymer. Medium, and possibly colloid stabilizer constitute initially an inhomogeneous system They are often preferred over solvent-based products in these applications due to the absence of volatile organic compounds (VOCs) in them.Įmulsion polymerization: Polymerization whereby monomer(s), initiator, dispersion These emulsions find applications in adhesives, paints, paper coating and textile coatings. A dispersion resulting from emulsion polymerization is often called a latex (especially if derived from a synthetic rubber) or an emulsion (even though "emulsion" strictly speaking refers to a dispersion of an immiscible liquid in water). In other cases the dispersion itself is the end product. Many of these polymers are used as solid materials and must be isolated from the aqueous dispersion after polymerization. When water-soluble polymers are used as stabilizers instead of soap, the repulsion between particles arises because these water-soluble polymers form a 'hairy layer' around a particle that repels other particles, because pushing particles together would involve compressing these chains.Įmulsion polymerization is used to make several commercially important polymers. The particles are prevented from coagulating with each other because each particle is surrounded by the surfactant ('soap') the charge on the surfactant repels other particles electrostatically.
These latex particles are typically 100 nm in size, and are made of many individual polymer chains.

Rather than occurring in emulsion droplets, polymerization takes place in the latex/ colloid particles that form spontaneously in the first few minutes of the process.

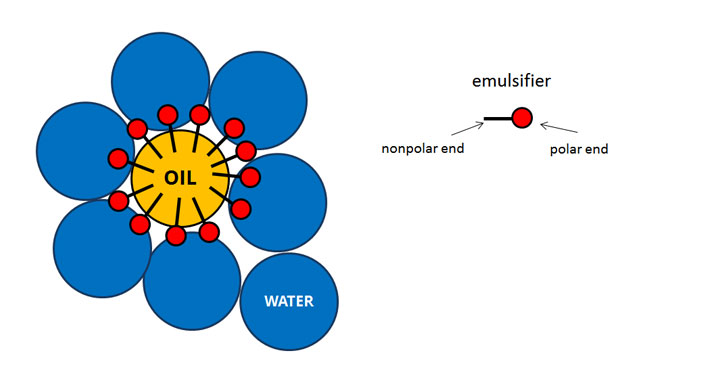
The name "emulsion polymerization" is a misnomer that arises from a historical misconception. Water-soluble polymers, such as certain polyvinyl alcohols or hydroxyethyl celluloses, can also be used to act as emulsifiers/stabilizers. The most common type of emulsion polymerization is an oil-in-water emulsion, in which droplets of monomer (the oil) are emulsified (with surfactants) in a continuous phase of water. Emulsion polymerization is a type of radical polymerization that usually starts with an emulsion incorporating water, monomer, and surfactant.


 0 kommentar(er)
0 kommentar(er)
